Vitrimers have ignited a revolution in the field of polymer chemistry with their remarkable ability to undergo dynamic and reversible reactions. This article takes you on an insightful journey into the fascinating realm of vitrimer chemistry, unveiling their versatile dynamic exchanges and their diverse applications.
1. Dynamic Transesterification and Related Exchanges
Vitrimer materials have ignited a revolution in the field of polymer chemistry with their remarkable ability to undergo dynamic and reversible reactions. This article takes you on an insightful journey into the fascinating realm of vitrimer chemistry, unveiling their versatile dynamic exchanges and their diverse applications.
1.1 Ester Bond Exchange
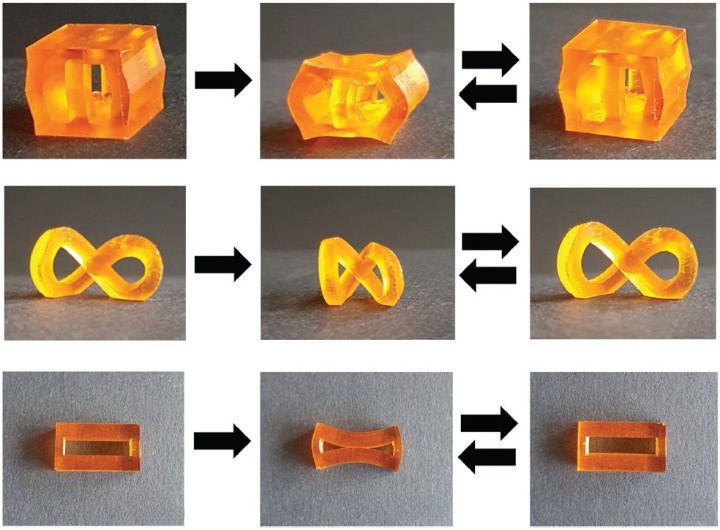
Ester bond exchange is a cornerstone of vitrimer chemistry. This dynamic reaction enables materials to undergo reversible changes in shape and form without compromising their structural integrity. The magic lies in the exchange of ester groups (–COOR) within the polymer structure.
When specific conditions, such as heat or the presence of catalysts, are met, these ester groups readily exchange places with neighboring ester groups, leading to the reshaping or repair of the material. Imagine a polymer that can heal itself when subjected to external forces or repair damage autonomously this is the promise of ester bond exchange vitrimers.
The applications of ester bond exchange vitrimers are diverse and far-reaching. In 3D printing, these materials offer the ability to create complex shapes and structures that can be reconfigured at will, reducing material waste. In the automotive industry, components made from ester bond exchange vitrimers can withstand repeated stress and repair minor damage, leading to longer-lasting and more reliable vehicles.
The sustainability and adaptability of ester bond exchange vitrimers make them a game-changer in materials science. As researchers continue to explore their potential, we can expect to see these materials revolutionize industries and contribute to a more sustainable and efficient future.
1.2 Carbamate and Carbonate Bond Exchange
Carbamate and carbonate bond exchange reactions are fundamental processes within vitrimer materials, contributing to their dynamic and adaptable properties. These reactions involve the reversible exchange of carbamate and carbonate groups within the polymer network.
Under specific conditions such as changes in temperature or the presence of catalysts, carbamate and carbonate bond exchange reactions allow vitrimers to adapt to varying environments and repair damage autonomously. This adaptability is highly desirable in applications where materials must withstand dynamic conditions or recover from mechanical stress.
Carbamate and carbonate bond exchange vitrimers find applications in diverse industries. In the realm of self-healing materials, they play a crucial role in enabling materials to recover from damage without external intervention. In recyclable composites, these vitrimers contribute to the reduction of waste and the promotion of sustainability in manufacturing processes.
The dynamic nature of carbamate and carbonate bond exchange reactions offers endless possibilities for innovative applications. As materials scientists continue to explore and harness their potential, we can expect to see carbamate and carbonate bond exchange vitrimers at the forefront of sustainable and adaptable materials development.
1.3 Carbamide (Urea) Bond Exchange
Carbamide, or urea, bond exchange reactions are a cornerstone of vitrimer chemistry, offering dynamic and reversible reshaping and repairing capabilities. These reactions involve the reversible exchange of urea groups within the polymer network, which can be triggered by specific conditions such as changes in temperature or the presence of catalysts.
Carbamide bond exchange vitrimers find applications in industries where adaptability and sustainability are paramount. The ability to change shape, repair damage, and recover autonomously aligns perfectly with the principles of eco-friendly manufacturing and reduced waste. This makes them highly valuable in applications such as flexible electronics and recyclable materials.
In the field of shape-memory materials, carbamide bond exchange vitrimers offer the ability to “remember” their original shape and return to it when subjected to specific conditions. This property is valuable in industries like healthcare and aerospace, where materials must adapt and respond to changing environments.
As researchers continue to explore the potential of carbamide bond exchange vitrimers, we can anticipate innovative solutions that address the challenges of sustainability and adaptability in materials science. These vitrimers hold the promise of a more efficient and eco-conscious future.
2. Dynamic Transacetalation and Transimination
Dynamic transacetalation and transimination reactions in vitrimers involve the exchange of acetal and imine bonds within the polymer network, respectively. These reactions are highly responsive to external stimuli, such as changes in pH or temperature, and play a crucial role in the adaptability of vitrimer materials.
2.1 Acetal Bond Exchange
Acetal bond exchange is a dynamic reaction that empowers vitrimers with the ability to reshape and repair autonomously. This process involves the reversible exchange of acetal groups within the polymer structure, triggered by factors such as changes in temperature or the presence of catalysts.
The applications of acetal bond exchange vitrimers are far-reaching. In self-healing materials, they can repair damage without external intervention, increasing the durability and reliability of products. In responsive coatings, these materials adapt to changing environmental conditions, making them valuable for applications where surface properties need to change dynamically.
As materials scientists delve deeper into the potential of acetal bond exchange vitrimers, we can anticipate breakthroughs in sustainability, durability, and adaptability across various industries.
2.2 Imine Bond Exchange
Imine bond exchange reactions are dynamic processes within vitrimers, enabling them to change shape, repair damage, or respond to specific conditions autonomously. This reaction involves the reversible exchange of imine groups within the polymer network, often triggered by changes in pH or temperature.
Imine bond exchange vitrimers are making strides in the field of shape-memory materials. They can “remember” their original shape and return to it when exposed to specific conditions, making them ideal for applications in healthcare and aerospace, where materials must adapt and respond to changing environments.
Moreover, imine bond exchange vitrimers find applications in adhesives, where dynamic reshaping and self-healing properties are highly desirable. These materials have the potential to revolutionize industries by providing sustainable, adaptable, and responsive solutions.
As materials scientists continue to explore the capabilities of imine bond exchange vitrimers, we can expect to see innovative applications that push the boundaries of sustainability and adaptability in materials development.
3. Dynamic Exchange of Conjugated Systems
The dynamic exchange of conjugated systems within vitrimer materials involves the reversible formation and breaking of bonds within conjugated systems, such as ketoenamines and thiol ethers. These exchanges contribute to the adaptability and responsiveness of vitrimer materials.
3.1 Ketoenamine Bond Exchange
Ketoenamine bond exchange reactions involve the reversible formation and breaking of bonds within ketoenamine groups within the polymer matrix. These exchanges can be triggered by changes in pH or temperature. Ketoenamine bond exchange vitrimers are valuable in applications requiring materials with dynamic shape-memory properties and responsive behavior.
3.2 Thiol Ether Conjugate Addition-Eliminations
Thiol ether conjugate addition-elimination reactions within vitrimers involve the reversible addition of thiol groups to alkene groups and subsequent elimination. These reactions are often initiated by external stimuli, such as heat or catalysts. Thiol ether conjugate addition-elimination vitrimers are highly responsive and adaptable, finding applications in fields like soft robotics and adaptive coatings.
4. Dynamic Transalkylation in Polyionic Systems
Dynamic transalkylation reactions within polyionic vitrimers involve the reversible exchange of alkyl groups within the polymer network. These exchanges contribute to the adaptability and responsiveness of vitrimer materials.
4.1 Transalkylation of N-centred Salts
Transalkylation of N-centred salts is a dynamic reaction within vitrimers that enables the reversible exchange of alkyl groups attached to nitrogen atoms. These exchanges can be initiated by external stimuli, such as changes in pH or temperature. Transalkylation of N-centred salts vitrimers are valuable in applications requiring dynamic responsiveness and adaptability, such as flexible electronics.
4.2 Transalkylation of Sulfonium Salts
Transalkylation of sulfonium salts within vitrimers involves the reversible exchange of alkyl groups attached to sulfur atoms. These exchanges are often triggered by changes in temperature or the presence of catalysts. Transalkylation vitrimers are highly adaptable and responsive, finding applications in industries requiring materials with self-healing and dynamic behavior, such as soft robotics.
5. Dynamic Heteroatom Bond Exchange
Dynamic heteroatom bond exchange within vitrimer materials involves the reversible formation and breaking of bonds with heteroatoms like boron (B), silicon (Si), and sulfur (S). These exchanges contribute to the adaptability and responsiveness of vitrimer materials in various applications.
5.1 Boronic Ester (B-O) Exchange
Boronic ester (B-O) bond exchange reactions involve the reversible formation and breaking of boronic ester bonds within the vitrimer structure. These exchanges are often initiated by changes in temperature or the presence of catalysts. Boronic ester exchange vitrimers find applications in industries requiring dynamic reshaping and self-healing properties, such as automotive components and advanced adhesives.
5.2 Silyl Ether (Si-O) Exchange
Silyl ether (Si-O) bond exchange reactions involve the reversible exchange of silyl ether groups within the vitrimer matrix. These exchanges can be triggered by specific conditions, such as changes in temperature or exposure to catalysts. Si-O bond exchange vitrimers exhibit dynamic adaptability, making them valuable in applications requiring responsive materials, such as drug delivery systems and adaptive coatings.
5.3 Disulfide (S-S) Bond Exchange
Disulfide (S-S) bond exchange reactions within vitrimer materials involve the reversible formation and breaking of disulfide bonds. These exchanges are often initiated by external stimuli, such as changes in temperature or the presence of catalysts. Disulfide bond exchange vitrimers are highly adaptable and responsive, finding applications in industries requiring materials with self-healing and dynamic behavior, such as aerospace components and structural materials.
6. Vitrimers with Multitype Dynamic Exchange Bonds
Vitrimers can be designed with multiple types of dynamic exchange bonds, enhancing their versatility and adaptability.
6.1 Vitrimers with Dual Dynamic Covalent Exchange Bonds
Vitrimers can incorporate dual dynamic covalent exchange bonds, combining the advantages of different exchange reactions within a single material. This design allows for tailored properties and enhanced performance, making them ideal for applications where versatility is crucial.
6.2 Vitrimers with Additional Non-Covalent Interactions
In addition to covalent bonds, vitrimers can feature non-covalent interactions, further expanding their dynamic behavior. These interactions can include hydrogen bonding, π-π stacking, and van der Waals forces, enabling responsive and adaptable materials for applications ranging from smart textiles to drug delivery systems.
In conclusion, vitrimer chemistry is a dynamic and rapidly evolving field with immense potential to revolutionize industries. By understanding the diverse dynamic exchange reactions and their applications, we can harness the power of vitrimers to create sustainable, adaptable, and responsive materials for a brighter future.